|
||
 |
Advanced modern transportation makes it easier for human to travel around the world and also, for pathogens. In addition, climate change accelerates the expansion of infectious agents and their transmissible vectors. It is important to clarify transmission mechanism and risk factors to control vector-borne diseases, while improving treatment and prevention. Recently, it is getting obvious that arthropod vectors are not only vehicles for pathogens, but actively involved with pathogen transmission via saliva affecting hosts’ physiological and immunological mechanisms. We are mainly working on the following projects. |
|||||
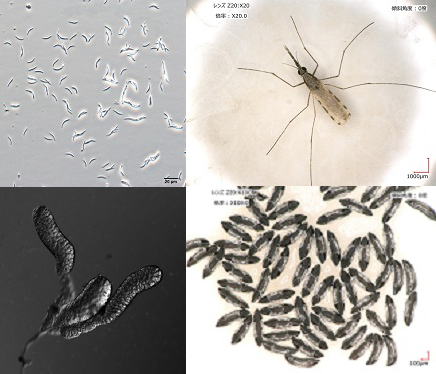
| | Molecular epidemiological study of leishmaniasis Leishmaniasis is caused by protozoan parasites of the genus Leishmania transmitted by tiny blood-sucking insects, phlebotomine sand flies,with a body length of approximately 2-3 mm. The disease is widely distributed around the world, especially in tropical and subtropical areas, affecting at least 12 million people in 98 countries. Approximately 20 Leishmania species are known to be pathogenic to humans, and species is the major determinant of clinical outcome (cutaneous, mucocutaneous and visceral forms). To date, more than 800 sand fly species have been recorded; of these, fewer than 10% have been confirmed as vector species of leishmaniasis. In addition, restricted species support the development of specific Leishmania species and consequently transmit the parasites. In this project, we establish molecular andserological tools for parasite identification, sand fly surveillance and reservoir research, and apply these methods to our field research on leishmaniasis. |
|||||
| References 1. Kato et al., PLoS Negl Trop Dis. 10: e0004844, 2016. 2. Kato et al., PLoS Negl Trop Dis. 10: e0004728, 2016. 3. Kato et al., PLoS Negl Trop Dis. 10: e0004336, 2016. 4. Koarashi et al., Acta Trop. 158: 83-87, 2016. 5. Nzelu et al., Acta Trop. 153: 116-119, 2016. 6. Kato et al., Acta Trop. 141: 79-87, 2015. 7. Gomez et al., Acta Trop. 137: 118-122, 2014. 8. Nzelu et al., Acta Trop. 132: 1-6, 2014. 9. Kato et al., Acta Trop. 128: 710-713, 2013. 10. Kato et al., Trans R Soc Trop Med Hyg. 105: 561-567, 2011. 11. Kato et al., Vector Borne Zoonotic Dis. 11: 515-521, 2011. 12. Kato et al., J Clin Microbiol. 48: 3661-3665, 2010. 13. Kato et al., Parasit Vectors. 3: 10, 2010. |
 |
||||
|
|
|||||
| | Study on salivary proteins from blood-feeding arthropods Blood-sucking insects have evolved a wide set of biologically active molecules to fight with host defense mechanisms. When probing in the host skin before blood sucking, they inject saliva, a cocktail of bioactive agents, containing anticoagulants, vasodilators, inhibitors of platelet aggregation, and anti-inflammatory agents. Since hematophagous arthropods have evolved their feeding strategies independently, each species has developed unique pharmacologically active agents in their saliva to overcome host hemostatic defenses. Interestingly, vector saliva is reported to enhance pathogen infection, indicating that vectors are not only vehicles for pathogens but also actively involved with pathogen transmission via saliva. On the other hand, specific immune response to the saliva provides protective immunity against pathogen infection, suggesting a novel vaccine strategy using vector saliva. In this project, we identified many novel molecules from saliva of blood-sucking insects, such as phlebotomine sand flies (vectors of leishmaniasis) and kissing bugs (vectors of Chagas disease). We are working to find out unique biological activities of these salivary components on host physiology and immunity, and elucidate mechanisms to affect pathogen infection and host defense. |
|||||
| References 1. Kato et al., Biochimie. 112: 49-56, 2015. 2. Kato et al., Infect Genet Evol. 13: 56-66, 2013. 3. Ishimaru et al., J Exp Biol. 215: 3597-3602, 2012. 4. Kato et al., Infect Genet Evol. 10: 184-191, 2010. 5. Hamasaki et al., J Insect Physiol. 55: 1044–1049, 2009. 6. Kato et al., J Exp Biol. 210: 733-740. 2007. 7. Kato et al., BMC Genomics. 7: 226. 2006. |
 |
||||
|
|
|||||
| | Study of Anopheles-Plasmodium interaction Mechanisms of blood feeding and
reproduction in mosquitoes Genetic manipulation of mosquitoes Malaria is one of the most important global public health issues. Malaria is caused by the malaria parasites, Plasmodium, and is transmitted by anophlienemosquitoes via blood feeding. Anopheline mosquito control as well as malaria vaccine development is one of the most effective ways to control malaria in endemic areas. Therefore, understanding of mosquito biology and malaria parasites in a mosquito stage is very important to develop new malaria control strategies. We are studying the mosquito biology, for example, the Anopheles-Plasmodium interaction, blood-feeding mechanisms in mosquitoes and mosquito reproductive biology. In these studies, we are using transgenesis andgenome-editing tools in anopheline mosquitoes and are performing functional analysis in vivo. Moreover, we are developing techniques to inhibit malaria infection in mosquitoes and to control fertility using genetically-modified (GM) mosquitoes. We are producing many GM mosquito lines, whereas the maintenance of these lines requires a large effort and cost. In mosquitoes, the efficient method for storage of GM lines is not established. Therefore, we are establishing the methods such as a cryopreservation, for GMlines in anopheline mosquitoes. |
|||||
| References 1. Yamamoto et al., PLoS Pathogens. 12: e1005872, 2016. press release 2. Yamamoto et al., Insect Mol Biol. 22: 685-693, 2013. 3. Yamamoto et al., J Exp Biol. 216: 2960-2966, 2013. 4. Sumitani et al., Insect Mol Biol. 22: 41-51, 2013. 5. Iyori et al., PLoS one. 8: e70819 2013. 6. Matsuoka et al., Trop Med Healt. 40: 47-52, 2012. 7. Yamamoto et al., Insect Mol Biol. 21: 223-233, 2012. 8. Yamamoto et al., Insect Mol Biol. 19: 391-398, 2010. 9. Hatakeyama et al., Proc Arthropod Embryo Soc Jpn. 44: 1-12, 2009. 10. Yamamoto et al., Mech Dev. 125: 996-1008, 2008. 11. 日本経済新聞記事掲載(平成28年7月17日23面) |
 |
||||
|
|
|||||
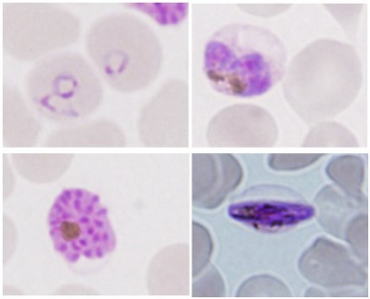
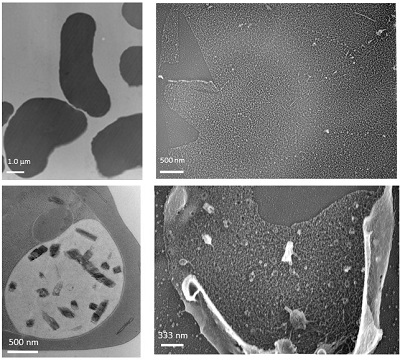
| | Protein-transport mechanism in Plasmodium falciparum-infected erythrocytes Malaria is one of the 3 major infectious diseases in worldwide and P. falciparummalaria is known to cause severe clinical cases. With recent global warming, habitats of mosquitoes have been shifted to northern area. Furthermore, malaria protozoa quickly obtain resistance against some anti-malaria drugs. Also, no effective malaria vaccine has been established so far. Taken those present situations, it is urgent to go further not only basic study for malaria but also create new medicine and vaccine as a translating researchin worldwide. After P. falciparum invades erythrocytes, over a couple of hundred kindsof proteins are transported to the surface of erythrocyte membrane. Some of the proteins are known to associate with serious clinical symptoms. However, still remaining question is how proteins are transported from the parasite that is enveloped in parasitized vesicle membrane (PVM), to erythrocyte membrane, even erythrocyte does not have nuclear. If protein transport mechanism will be clarified, this mechanism will be a potential target to development for new clinical treatment and drugs. I have been focusing Maurer’s cleft (MC), which is only constructed in the cytosol of P. falciparum-infected erythrocytes, and considered to have functions as“carrier and/or transport pathway”for parasite proteins. To clarify these possibilities, I have been studying 3 dimensional structure of MC by transmission electron microscope (TEM) and the mechanism of their formation, involving lipid metabolism by biochemical technique, etc. Recently, critical 3D-MC structures were successfully observed by unique technique termed “unroofingtechnique”which exposes P. falciparum-infected erythrocytes cytosol before TEM observation. Other topics involve comparative studies ofphysico-chemical aspects of infected-erythrocytes between human malaria and other non-human species (mouse, avian). Those results will be effective for new knowledge to further develop clinical treatment and drugs for human malaria. References 1. Hayakawa et al., Exp Parasitol. 153: 174–179, 2015. 2. Hayakawa et al., BioMed Res Int. 2015: 642729, 2015. |
|||||
 |
 |
||||
|
|
|||||
| | Role of cell membrane / organelle membranes on infectious diseases Most infections caused by virus, bacteria and protozoa occur via host cell membranes. Many recent studies reported; (1) lipid microdomain(or raft) of host cell membrane is required to complete the infection processes, and (2) cholesterol, one of the major components of lipidmicrodomain/raft, modulates the activity of membrane proteins responsible for the intracellular and extracellular signal transduction, exocytosis, endocytosis. Thus, it is important to investigate the function and physico-chemical aspects of cell / organella membrane in a variety of infectious diseases. For example, Mycobacterium tuberculosis (MTB) can survive in phagosome in macrophage by interfering phagosome-lysosome membrane fusion. I found that Lipoarabinomannan, one of the MTB derived-glycolipid, stabilized the cholesterol-sphingomyelin domain structures in liposome membranes and inhibited membrane fusion. These results suggested a possible mechanism for MTB to escape anti-bacteria processes in lysosomes by inhibiting phagosome-lysosome membrane fusion. Also, I studied many physical aspects of parasitized erythrocytes, including modifications of cytoskeleton structure, lipid distribution of erythrocytes, and lipid-protein interactions. Furthermore, I reported erythrocytes with different hemoglobin type showed different patterns of raft domain in erythrocyte membrane. Based on these studies, I am now carrying out some projects; lipid metabolism of malaria protozoa, lipid import mechanism from environments, structure and function of exo-membranes constructed in parasitized erythrocytes using a variety of biophysical, biochemical, and cell physiological techniques. Also, I am developing a new imaging technique using Ion Liquid and sample preparation protocols optimized for high-resolution electron microscopy imaging. |
|||||
| References 1. Hayakawa et al., Biophys J. 93: 4018–4030, 2007. 2. Tokumasu et al., PLoS ONE. 4: e5828 3. Hayakawa et al., PLoS ONE. 8: e85467 4. Hayakawa et al., BioMed Res Int. 2015: 642729, 2015. 5. Hayakawa et al., Parasitol Int. 65: 539-544, 2016. 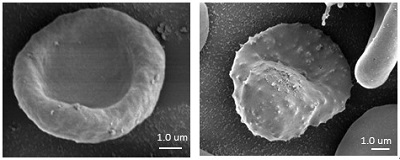 |
 |
||||
|
|
|||||
| | Study on the mechanism restricting vector competence in
hematophagous arthropod midgut
Leishmaniasis and malaria, vector-borne diseases,
is caused by protozoan parasite of the genus of Leishmania and Plasmodium,
respectively. The diseases are spread by hematophagous arthropod such as sand
fly and mosquito. Although the vector competence has been thought to depend on the
species-specific interaction, the
mechanism restricting vector competence is poorly understood. The parasites
develop into infective form for human in the midgut of vector. Midgut is not
only plays an important role in nutrition absorption, but also, is the immuno-modulating
environment for maintaining the beneficial microbiota composition, similar with
human. We are working to unveil the mechanism restricting vector competence, focusing
on immune response, microbiota, physiology of parasite in the vector midgut. |
|||||
| Copyright@Division of Medical Zoology, Department of Infection and Immunity,
Jichi Medical University all right researved. No reproduction or republication without written permission. |
|||||